In science, accuracy and precision are paramount. Whether performing experiments in chemistry, physics, or biology, why do scientists use significant figures? Significant figures are essential tools for ensuring that measurements are accurately communicated and interpreted. They reflect the precision of measurements and help scientists avoid overstating or understating the reliability of their data.
Significant figures play a crucial role in scientific calculations. When measurements are made, they carry a certain level of uncertainty based on the tools and methods used. Scientists use significant figures to report the data with the proper level of precision, ensuring the results are meaningful and consistent across experiments. By understanding and applying the rules of significant figures, scientists can ensure that their conclusions are based on reliable data and avoid misleading results.
This article will explore the importance of significant figures, their role in scientific calculations, and how they contribute to the reliability and consistency of scientific work.
Why do scientists use significant figures?
Scientists use significant figures to indicate the precision of measurements and calculations. By following the rules for significant figures, they ensure their results are accurate, consistent, and reliable. This prevents the overstatement of precision and maintains the integrity of scientific data, which is essential for reproducible experiments and meaningful conclusions.
The Basics of Significant Figures and Their Role in Precision
Significant figures are the digits in a number that carry meaningful information about its precision. They are essential in scientific measurements because they allow scientists to express the accuracy of their measurements clearly.
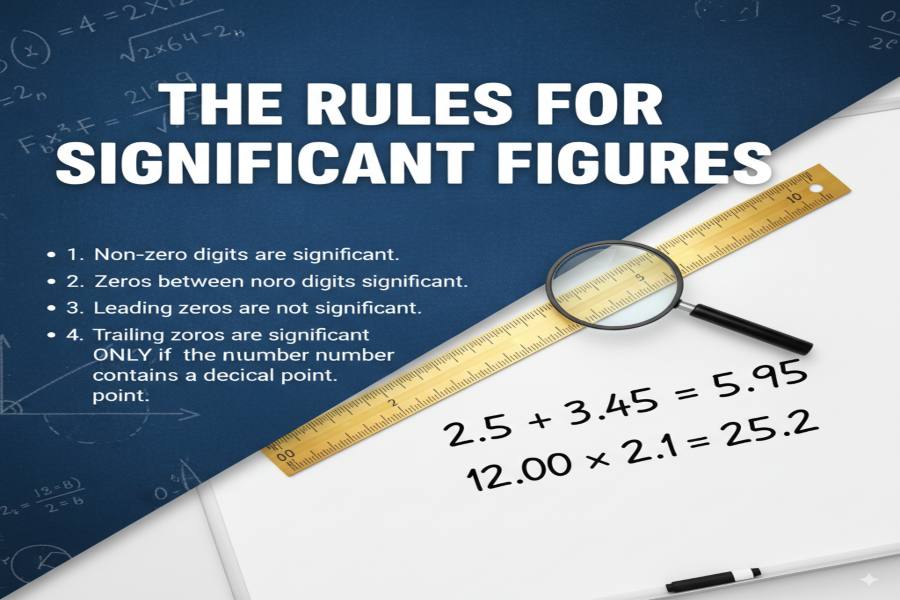
To understand significant figures, it’s essential first to know the basic rules for counting them:
- Non-zero digits: All non-zero digits are always significant. For example, in the number 123.45, all digits are significant.
- Zeros between non-zero digits: Zeros between non-zero digits are also significant. For instance, 1002 has four significant figures.
- Leading zeros: Zeros to the left of the first non-zero digit are not significant. For example, 0.00457 has three significant figures, as the leading zeros don’t count.
- Trailing zeros in a decimal number: Trailing zeros in a number with a decimal point are significant. For example, 12.00 has four significant figures.
- Trailing zeros in a whole number without a decimal point: These are not significant. For example, 1200 only has two significant figures.
By following these rules, scientists can communicate the precision of their measurements and avoid overestimating their results.
The Application of Significant Figures in Scientific Calculations
Significant figures are crucial in ensuring the precision of calculations, and understanding how to apply them in different operations is essential for accurate scientific results. Let’s look at how significant figures are applied in various mathematical operations.
Addition and Subtraction:
When adding or subtracting, the result should be rounded to the least number of decimal places. For example:
- 12.11 + 5.5 = 17.6 (The least precise number is 5.5, so the result is rounded to one decimal place).
Multiplication and Division:
In multiplication and division, the result should have the same number of significant figures as the number with the fewest significant figures in the operation. For instance:
- 4.56 × 2.1 = 9.6 (Since 2.1 has two significant figures, the result is rounded to two significant figures).
Scientific Notation:
Significant figures also play a vital role in scientific notation. The precision of the number is indicated by the significant figures, no matter how large or small the number is. For example, 5.20 × 10^3 has three significant figures.
By adhering to these rules, scientists ensure that their calculations are consistent and accurately reflect the limitations of the measurements.
Why Are Significant Figures Essential for Scientific Research?
In scientific research, the importance of significant figures cannot be overstated. They ensure that data is communicated in a way that accurately reflects its precision, which is critical for drawing valid conclusions and making informed decisions.
Key reasons scientists use significant figures in research:
- Accurate reporting of data: Significant figures ensure that measurements are reported in a way that aligns with the precision of the instruments used.
- Consistency: By applying the rules of significant figures consistently, scientists can compare different studies and experiments without risking misinterpretation of the data.
- Reliability: Correctly using significant figures ensures that results are reproducible, which is essential in scientific inquiry.
When significant figures are correctly applied, they help eliminate the possibility of misleading results and ensure the validity of scientific conclusions.
Common Errors in Applying Significant Figures and How to Correct Them
In this section, we’ll explore common mistakes made when applying significant figures and how to avoid them.
- Overestimating precision: Often, people report more significant figures than their instruments can measure. This can lead to inflated precision and misinterpretation of results. To correct this, always match the number of significant figures to the precision of your measuring tool.
- Incorrect rounding: Sometimes, rounding occurs too early or inconsistently, which can distort final results. To avoid this, round only at the final step of a calculation.
- Ignoring significant figures in scientific notation: Some people forget to apply the correct significant figures when dealing with numbers in scientific notation. Always ensure that scientific notation maintains the same level of precision as the original measurement.
By recognizing these common errors and following the rules carefully, scientists can maintain the integrity of their data and avoid misleading conclusions.
How Significant Figures Influence Data Interpretation in Science?
Significant figures play a crucial role in the interpretation of scientific data. They help ensure that data is presented clearly and accurately, providing insight into the precision behind measurements. Without correct application, significant figures can lead to misinterpretations and flawed conclusions.
How significant figures affect interpretation:
- Misleading conclusions: Overreporting precision can cause scientists to believe that their results are more accurate than they actually are.
- Consistency in data comparison: Proper use of significant figures ensures that results from different experiments or studies are comparable and trustworthy.
- Reproducibility: Ensuring that results reflect the correct level of precision allows experiments to be reproduced accurately by other researchers, a cornerstone of scientific validity.
By understanding how significant figures impact data interpretation, scientists can present their findings in a clear and precise manner.
Final Thoughts
In conclusion, why do scientists use significant figures? They are essential for ensuring precision and accuracy in scientific measurements and calculations. By following the rules for significant figures, scientists can report their findings consistently and avoid overestimating precision. Whether in chemical reactions, physical measurements, or laboratory experiments, significant figures help ensure that results are reliable, reproducible, and accurate. Their proper use is crucial for maintaining the integrity of scientific data, preventing errors, and allowing for valid comparisons across studies. Understanding and applying significant figures is key to producing meaningful scientific work that contributes to the advancement of knowledge in various fields.
FAQ’s
What are significant figures?
Significant figures are digits in a number that indicate the precision of a measurement. They help convey how accurately a number is measured or calculated.
Why is it essential to use significant figures in science?
Significant figures ensure that data is communicated accurately, reflecting the precision of the measurement tools used and preventing overstatement of precision.
How do you apply significant figures in calculations?
For addition and subtraction, round to the least decimal place. For multiplication and division, round to the least number of significant figures in the data.
Can significant figures affect scientific results?
Yes, improper use of significant figures can lead to inaccurate results, misinterpretation of data, and flawed conclusions.
How do significant figures affect reproducibility in science?
By accurately reporting measurements and calculations with the correct number of significant figures, scientists ensure that others can reliably reproduce experiments.