In chemistry, precision is paramount when making measurements and calculations, and this is where significant figures come into play. What are significant figures in chemistry? Significant figures represent the meaningful digits in a measurement, indicating the level of precision. When conducting experiments, it’s crucial to report measurements accurately, and the use of significant figures ensures that the data is not overstated or understated in terms of precision.
In scientific calculations, such as those performed in chemistry, significant figures help avoid misrepresentation of results. For example, when adding or multiplying values, the number of significant figures in the final answer must reflect the precision of the least precise value in the calculation. This helps maintain the integrity of data reporting, ensuring consistency and meaningfulness.
Understanding how to apply significant figures correctly in various calculations—whether adding, subtracting, multiplying, or dividing—is essential for chemistry students and professionals alike. By adhering to the rules of significant figures, chemists ensure the accuracy and reliability of their results, ensuring their findings are trustworthy for further analysis and application.
What are significant figures in chemistry?
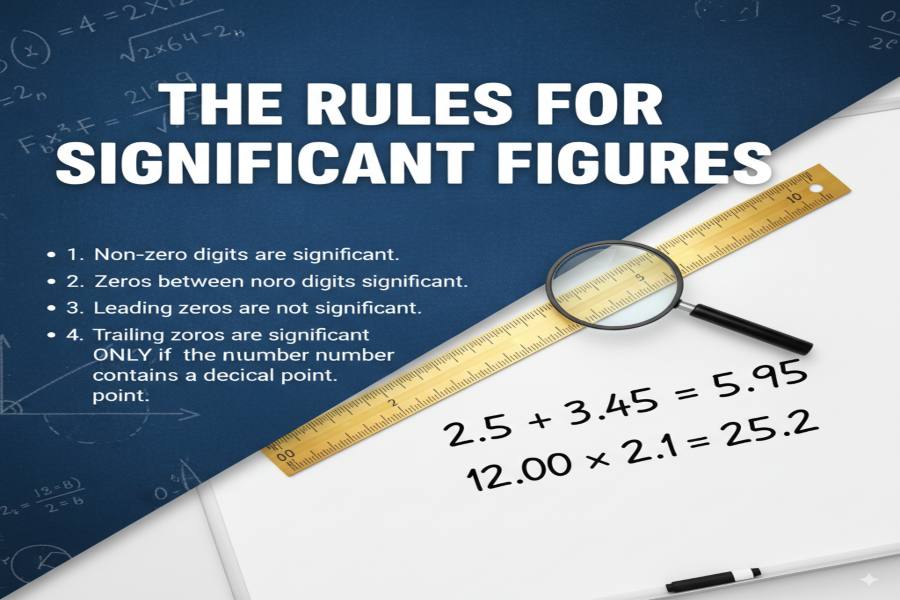
In chemistry, significant figures are the digits in a measurement that reflect its precision. They include all non-zero digits, any zeros between them, and trailing zeros in the decimal portion of the number. Significant figures help chemists report measurements accurately, ensuring consistency in calculations, such as in chemical reactions and stoichiometry, and avoiding overstatement of precision.
The Importance of Significant Figures in Chemistry for Accurate Results
In chemistry, significant figures are vital for ensuring precision in measurements and calculations. Significant figures represent the digits in a number that are meaningful and convey the level of precision of the measurement. Understanding how to use significant figures properly is essential for accurate data reporting in scientific experiments.
To begin, significant figures include all non-zero digits, any zeros between them, and trailing zeros in decimal numbers. Leading zeros are not considered significant. Knowing these basic rules helps chemists accurately communicate the reliability of their measurements. For instance, if you measure the concentration of a substance or the volume of a liquid, the number of significant figures indicates how precise the measurement is.
In chemical calculations, such as stoichiometry or reaction rate calculations, significant figures ensure that results are not overstated in terms of precision. If the measurement tool’s accuracy is limited, the result should reflect that limitation. By adhering to the rules of significant figures, chemists can avoid errors and ensure their findings are both reliable and reproducible, maintaining the integrity of the scientific work.
Why Are Significant Figures Crucial in Chemical Calculations?
This section will explore why significant figures are vital in chemical calculations, particularly in experiments and research. When working with chemical reactions, proper use of significant figures ensures that results are communicated clearly and without exaggeration.
The role of significant figures in chemical calculations:
- Precision in reactions: Accurate measurements are crucial for chemical reactions, such as determining reactant amounts or calculating product yields.
- Consistency in stoichiometry: Stoichiometric calculations rely on significant figures to ensure that conversion factors and measured quantities are consistent, providing reliable results.
- Data interpretation: The accurate use of significant figures enables chemists to interpret experimental data and compare results, ensuring consistency in research.
Common Mistakes in Using Significant Figures in Chemistry
In this section, we’ll highlight some common mistakes students and professionals make when applying significant figures in chemistry. Understanding these errors can help prevent inaccurate results in calculations.
Mistakes to avoid:
- Overestimating precision: Reporting more significant figures than the measurement allows.
- Misapplying rules in operations: Failing to round correctly when adding, subtracting, multiplying, or dividing values.
- Ignoring significant figures in scientific notation: Not correctly applying significant figures when working with large or small numbers in scientific notation.
How Significant Figures Work in Chemical Operations?
In this section, we’ll explore how to apply significant figures in various chemical operations, ensuring accurate results in experiments. Understanding how to use significant figures properly is crucial for chemical calculations. Here’s how they work in different operations:
- Addition and Subtraction: When adding or subtracting numbers, the result must be rounded to the least number of decimal places among the values involved. This ensures that the result reflects the precision of the least precise measurement.
- Multiplication and Division: For multiplication and division, the result should have the same number of significant figures as the measurement with the fewest significant figures. This rule prevents over-reporting precision and maintains consistency in calculations.
- Scientific Notation: Significant figures are significant in scientific notation, especially when working with very large or tiny numbers. They indicate the level of precision in these values, helping to maintain clarity when expressing measurements in a compact form.
By applying these rules, chemists ensure that their data is accurate, reliable, and appropriately represented in their calculations.
Real-Life Examples of Significant Figures in Chemistry
In chemistry, significant figures are crucial for ensuring precision and accuracy in various real-life applications. One typical example is in the preparation of chemical solutions. When measuring the concentration of reagents, it’s essential to use the correct number of significant figures to ensure the reaction proceeds as expected. If the concentration is reported with too many or too few significant figures, it could lead to inaccurate results, affecting the success of the experiment.
Similarly, in chemical kinetics, significant figures are vital for determining reaction rates. Accurate measurements of the time taken for a reaction and the amount of reactant consumed require proper use of significant figures. This ensures that the calculated reaction rate is precise and reliable, allowing chemists to make valid comparisons between different reactions.
In industrial processes, significant figures are just as important. For example, when producing chemicals at large scales, maintaining precision in measurements ensures product consistency and prevents waste. Overall, significant figures play a crucial role in maintaining the integrity and accuracy of chemical work in both laboratories and industries.
In Summery
In conclusion, what are significant figures in chemistry? They are crucial for ensuring the precision and accuracy of measurements and calculations in chemical experiments. By following the rules of significant figures, chemists can accurately convey their findings without overstating the precision of their results. Whether it’s stoichiometric calculations, reaction rate analysis, or solution preparation, understanding significant figures is vital for producing reliable and reproducible results. They help maintain the integrity of scientific data, ensuring that conclusions drawn from experiments are based on realistic, precise measurements. Mastering significant figures is a key part of chemistry that enables accurate data reporting and interpretation.
FAQ’s
What are significant figures in chemistry?
Significant figures are digits in a number that provide meaningful information about the precision of a measurement, ensuring accurate data reporting.
Why are significant figures important in chemistry?
They are essential for maintaining accuracy and consistency in chemical measurements, calculations, and experiments, thereby ensuring the reliability of the results.
How do you count significant figures in chemistry?
Non-zero digits are always significant, as well as any zeros between non-zero digits or after a decimal point. Leading zeros are not significant.
What happens if significant figures are misused in chemistry?
Misuse can lead to inaccurate results, misinterpretation of data, and potentially flawed conclusions, affecting the reliability of scientific work.
How do significant figures affect chemical reactions?
Accurate use of significant figures ensures that the measurements of reactants and products in chemical reactions are precise, leading to correct results in stoichiometry and other calculations.