In chemistry, physics, and other scientific fields, precision in measurements is crucial. What are the rules for significant figures, and why are they so important? Significant figures are the digits in a number that convey meaningful information about its precision. Using them correctly ensures that measurements are reported with the appropriate level of accuracy, avoiding the overstatement of precision.
The rules for significant figures help us communicate the accuracy of a measurement. For example, in a measurement like 0.00457, the number of significant figures reflects the precision of the measurement tool used. In calculations, understanding how to apply these rules ensures the results are consistent with the input data. Whether performing simple arithmetic or complex scientific calculations, adhering to significant figure rules ensures that you don’t mislead others or yourself with false precision.
In this article, we will explore the rules of significant figures, explain their importance in scientific work, and provide examples to help you apply them correctly in various mathematical operations.
What are the rules for significant figures?
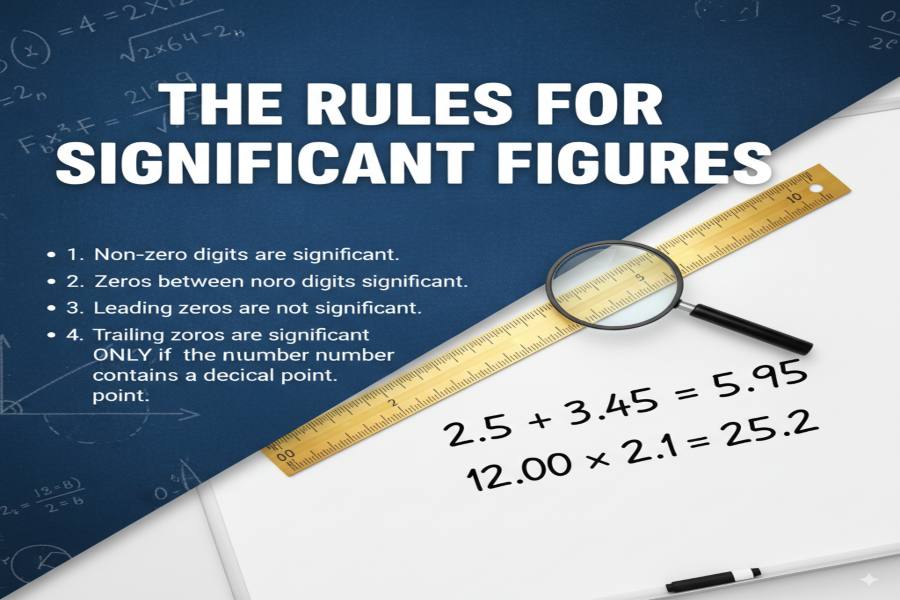
The rules for significant figures are guidelines used to determine which digits in a number are meaningful and contribute to its precision. The main rules include counting non-zero digits, any zeros between significant digits, and trailing zeros in decimal numbers. For addition and subtraction, round the result to the least number of decimal places, while in multiplication and division, the result should have the same number of significant figures as the least precise number in the calculation.
Mastering Significant Figures and Their Rules for Accuracy
In this section, we will define significant figures and explore their role in ensuring precise data communication in scientific measurements. Significant figures are the digits in a number that convey meaningful information about its precision. Understanding the rules for counting significant digits, handling zeros, and applying scientific notation is crucial for accurate reporting.
The basic rules for significant figures include counting all non-zero digits as significant, recognizing zeros between significant digits as meaningful, and handling trailing zeros correctly in decimal numbers. For example, in a number like 0.00457, the digits 4, 5, and 7 are significant, while the leading zeros are not. Similarly, in scientific notation, significant figures maintain their meaning, regardless of the size of the number.
By mastering these rules, you ensure that measurements are reported with the correct level of precision. Overestimating the precision of measurements can mislead others, particularly in experiments or chemical reactions where the accuracy of tools and methods is crucial. Using too many significant figures can imply false precision, leading to errors in data interpretation and potentially flawed conclusions. This section will equip you with the knowledge to apply significant figures correctly in all contexts.
When and Why Significant Figures Are Used?
In this section, we will focus on the importance of significant figures in scientific calculations and measurements. Understanding when and why to use them helps ensure accurate data reporting and prevents overstating precision.
- Avoiding overestimated precision: Proper use of significant figures ensures that measurements are not misrepresented as more precise than the instruments allow. This helps maintain the integrity of the data.
- Ensuring reliable results in experiments: By using the correct number of significant figures, scientists can avoid misleading results in experiments, particularly in chemical reactions. This ensures that conclusions drawn are based on accurate, reliable data.
- Reporting data consistently: Significant figures help maintain consistency across scientific studies, making it easier to compare results from different experiments. Accurate and consistent data reporting is essential for reproducibility and trust in scientific findings.
By following the rules for significant figures, scientists can ensure that their results are meaningful, reliable, and accurate, ultimately supporting valid conclusions and further research.
Common Mistakes When Using Significant Figures
This section will highlight common errors people make when using significant figures and how to avoid them. Using incorrect significant figures can lead to inaccurate results and misinterpretations, so it’s essential to be aware of potential pitfalls.
Common mistakes:
- Overuse of significant figures: Reporting more figures than the measurement tool allows.
- Rounding incorrectly: Failing to round results properly based on the rules of significant figures.
- Ignoring scientific notation rules: Not applying significant figures correctly when working with very large or tiny numbers.
How Significant Figures Work in Mathematical Operations?
Significant figures play a crucial role in ensuring the accuracy of mathematical operations, such as addition, subtraction, multiplication, and division. Understanding how to apply the rules for significant figures in these operations is key to maintaining precision in scientific calculations.
Addition and Subtraction
When adding or subtracting numbers, the result should be rounded to the least number of decimal places among the numbers involved. This rule ensures that the final answer reflects the precision of the least precise measurement, preventing over-reporting of accuracy.
Multiplication and Division
In multiplication and division, the number of significant figures in the result should match the number of significant figures in the measurement with the fewest significant figures. This ensures that the final result does not imply more precision than the original measurements allow.
Scientific Notation
Significant figures are also important in scientific notation, especially when dealing with very large or tiny numbers. They help maintain the precision of the number, ensuring that the result accurately reflects the measurement’s accuracy, regardless of its size.
Real-Life Applications of Significant Figures in Science
In this section, we will discuss real-world scenarios where understanding the rules for significant figures is essential. These applications include chemical reactions, laboratory experiments, and industrial processes.
- In laboratory experiments: Accurate use of significant figures ensures precise measurement of reagents, concentrations, and volumes, which are critical for successful reactions.
- In chemical calculations: When calculating reaction yields, stoichiometry, or molarity, significant figures help maintain the precision of the calculations.
- In industry, significant figures help ensure consistency and quality control in product production, particularly for processes such as manufacturing or pharmaceuticals.
Final Remarks
nderstanding the rules for significant figures is essential for ensuring accuracy and precision in scientific measurements and calculations. Whether conducting experiments, analyzing data, or performing chemical calculations, adhering to these rules ensures consistent and meaningful results. Correctly applying significant figures helps prevent errors, avoids overstating precision, and maintains the integrity of scientific work. By following these guidelines, scientists and researchers can produce reliable, reproducible results, making their findings trustworthy and comparable. Mastering the rules of significant figures is crucial for clear communication in science and for maintaining the credibility of experimental outcomes.
FAQ’s
What are significant figures in measurements?
Significant figures are the digits in a measurement that reflect its precision. They include all non-zero digits, any zeros between significant digits, and trailing zeros in decimal numbers.
Why is it important to follow the rules for significant figures?
Following these rules ensures that measurements are accurately reported, preventing the false impression of precision and ensuring consistent and reliable calculations.
How do significant figures apply in scientific notation?
In scientific notation, significant figures indicate the precision of very large or tiny numbers. The significant figures in scientific notation help clearly express the accuracy of the number.
What happens if you ignore the rules for significant figures?
Ignoring these rules can lead to misleading results and errors in calculations, affecting the accuracy and reliability of scientific work.
How do I round numbers according to significant figures?
In addition and subtraction, round to the least number of decimal places; in multiplication and division, round to the least number of significant figures in the original numbers.