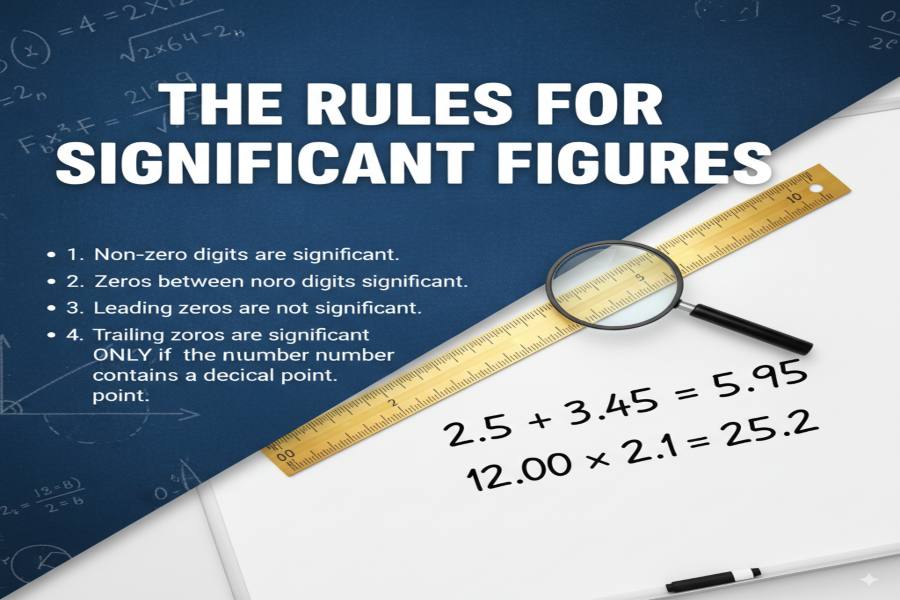
Significant figures play a crucial role in the world of mathematics, science, and engineering. They represent the precision of a measured or calculated value and are essential for ensuring that data is accurately communicated and interpreted. In practical terms, significant figures help prevent over-exaggeration of the precision of measurements, offering a better understanding of how reliable a result is.
For instance, when reporting measurements in a scientific experiment, it’s essential to show the precision with which those values were obtained. Overstating or understating precision can lead to misleading results or conclusions. Whether you’re calculating the mass of an object, the speed of a car, or the distance between planets, knowing how to apply significant figures allows scientists, engineers, and even students to present their results clearly and consistently.
Understanding the importance of significant figures ensures you make better decisions in data interpretation, avoid false conclusions, and uphold the integrity of your work. In this article, we’ll delve deeper into why significant figures are so vital in various fields and how they contribute to precise and reliable measurements.
Why are significant figures important?
Significant figures are crucial because they convey the precision of a measurement. Using them correctly ensures that data is accurate, reliable, and avoids misleading interpretations. In science, they indicate the precision of a measurement, helping to maintain consistency in calculations and experiments.
The Importance of Significant Figures in Achieving Accuracy
Significant figures are essential for ensuring the accuracy of scientific measurements. Since measurements are rarely perfect, significant figures help convey the level of precision in those values, preventing the presentation of misleadingly exact results. By using the correct number of significant figures, we can accurately communicate the precision of measurements without overestimating their reliability.
When performing calculations such as addition, subtraction, multiplication, and division, the number of significant figures in the result should match the least precise number involved in the operation. For example, when multiplying two values with different precisions, the result must reflect the precision of the least precise value. This ensures that the final result maintains integrity and accurately reflects the precision of the data.
In scientific disciplines such as chemistry and physics, using significant figures correctly is crucial for producing reliable reports and consistent results. Misapplying significant figures can lead to errors, miscommunication, or flawed conclusions. Whether in laboratory experiments or daily measurements, significant figures provide clarity and consistency, ensuring data is interpreted and communicated accurately, which is vital for further research, practical applications, and avoiding costly mistakes.
How Significant Figures Improve the Reliability of Calculations?
The use of significant figures is not just a mathematical convention; it improves the reliability of calculations by ensuring that data is not exaggerated or underreported. When performing calculations in experiments, the outcome must be rounded based on the significant figures of the inputs, which maintains the integrity of the final result.
Key Points:
- Why significant figures are essential for precise calculations: Discuss the role of significant figures in maintaining consistency and accuracy in scientific work.
- How to apply significant figures when adding or subtracting values: Different rules apply when dealing with these operations compared to multiplication and division. This section will focus on how you can maintain precision during basic mathematical operations.
- The role of significant figures in scientific notation: Use examples to explain how significant figures work in scientific notation and why this is essential for clarity and consistency in reporting.
Practical Examples of Significant Figures in Action
In this section, we’ll provide practical examples that demonstrate the importance of significant figures in everyday situations.
For example, consider a simple measurement like the length of a pencil. If you measure the pencil as 15.3 cm, it means the measurement is precise to one decimal place, implying that the pencil’s length is accurate up to the tenth place.
- Practical example of using significant figures in chemistry
- Significant figures in physics experiments
- Everyday usage, like measuring the temperature or speed
Bullet points:
- Measurement: Always round the results of calculations based on the least number of significant figures from the input.
- Chemistry example: Consider a reaction rate measured with a high degree of precision, and the importance of significant figures in presenting this result.
- Physics example: Measuring the velocity of an object with a radar gun, emphasizing how significant figures help communicate the measurement’s accuracy.
How to Round Numbers with Significant Figures?
Rounding numbers accurately is a crucial skill when working with significant figures. It ensures the precision of measurements is maintained without overstating the accuracy. The rounding rules depend on the type of operation being performed. For example, when adding or subtracting numbers, the result should be rounded to the least number of decimal places among the numbers involved. This ensures that the result is not presented with more precision than the original data.
For multiplication and division, the result must be rounded to match the least number of significant figures from the numbers being multiplied or divided. This rule helps avoid exaggerating the precision of the result and ensures that calculations are consistent with the original measurements.
In this section, we’ll discuss how to correctly round numbers when using significant figures, highlighting common mistakes like rounding too early or inaccurately. By following the appropriate rounding rules, you’ll preserve the integrity of your data while avoiding misrepresentations.
Why Significant Figures Matter in Scientific Research?
In scientific research, accurate data is crucial for developing theories, making predictions, and advancing technologies. Understanding how to apply significant figures properly is vital for ensuring the reliability and credibility of research outcomes. By adhering to the rules of significant figures, researchers can communicate their findings with clarity, ensuring that results are both reproducible and trustworthy.
Significant figures help define the precision of experimental results, preventing overstatements of accuracy. They ensure that data is reported with the appropriate level of detail, avoiding the false impression that a measurement is more precise than it truly is. This is especially important when interpreting and comparing experimental results, as it ensures consistency and reduces the risk of errors.
In research, using significant figures correctly allows scientists to present their data in a manner that accurately reflects the actual limitations of their measurements. This transparency not only upholds the integrity of the research but also fosters greater confidence in the conclusions drawn from the data.
Conclusion
Significant figures are much more than just a technicality in mathematics and science; they are essential for ensuring the accuracy and integrity of data reporting. Whether you’re in the laboratory, classroom, or simply measuring something in your daily life, understanding how to use significant figures properly helps ensure that your results are not exaggerated or misleading.
By respecting significant figures, scientists and researchers can maintain the reliability of their findings, thereby advancing knowledge and building upon previous work. Their importance cannot be overstated, as they allow us to communicate with precision and clarity in the fields of science, engineering, and beyond.
FAQ’s
What are significant figures?
Significant figures are digits in a number that carry meaningful information about its precision. They help ensure that measurements and calculations are reported accurately.
Why is it essential to use significant figures?
Using significant figures ensures that the precision of measurements is communicated accurately, preventing overstatements or misinterpretations in data.
How do I determine the number of significant figures in a number?
Count all non-zero digits, any zeros between them, and trailing zeros in the decimal portion of a number.
What happens if I use too many significant figures in my calculations?
Using too many significant figures can give the false impression that the measurement is more precise than it actually is, leading to errors in interpretation.
How do significant figures apply to scientific notation?
Significant figures in scientific notation follow the same rules as standard notation, helping to indicate the precision of the measurement, especially when dealing with extremely large or small numbers.